Charles Law
Jacques Charles
(November 12, 1746 – November 7, 1823)
Jacques Charles was born in Beaugency sur Lorie in 1746, he was married to Julie Francoise Bouchaud des Herettes, she was 37 years younger than charles. He was thought to be very personally intellectual with the opposite sex as well. Charles and his assistance the Robert brothers made the worlds first hydrogen filled baloon in august 1783.
Charle's Law is known as the law of volumes states how gases expand when they are heated in constant temperature.
In 1787 Charles did an experiment where 5 balloons were filled with the same volume but with different gases and were all raised to a constant temperature of 80 °C. He saw that they all increased in volume by the same amount. This just show us the relationship between volume and temperature of a gas. Charles' Law describes that under constant pressure, a gas volume is proportional to its absolute temperature. The gas volume under constant pressure will increase linearly with the temperature of the gas. The formula he created was V1/T1 = V2/T2.
Charles created a law known as the charles law on gas. It explains how the gas expands when it's heated. Also at constant pressure, the volume (Gas) will increases or decreases relative to the temperture, depending on weather the temperture will increase or decrease. For example if the temerture increased the gas will expand.
which can be written as:
Charles created a law known as the charles law on gas. It explains how the gas expands when it's heated. Also at constant pressure, the volume (Gas) will increases or decreases relative to the temperture, depending on weather the temperture will increase or decrease. For example if the temerture increased the gas will expand.
which can be written as:


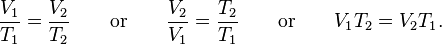

No comments:
Post a Comment